The development of robust and scalable synthetic routes remains a critical challenge in modern process chemistry, particularly when transitioning from laboratory concepts to industrial production. Recent work on a novel route to 8-aminooctanoic acid demonstrates how careful process design and step integration can enable reliable scale-up without compromising efficiency or selectivity.
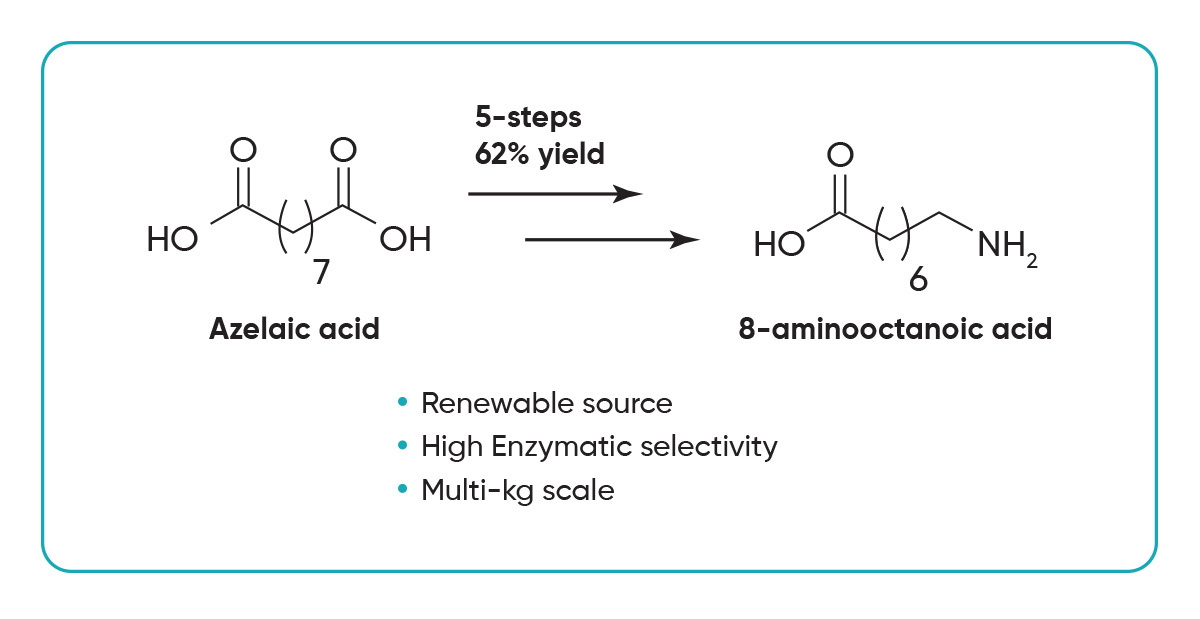
Starting from commercially available azelaic acid, the target compound is obtained in four well-defined steps with an overall yield of 62%. The process begins with a Fischer esterification, followed by a highly selective enzymatic hydrolysis to produce methyl hydrogen azelate. Subsequent ammonolysis using aqueous ammonia and a Hofmann rearrangement yield 8-aminooctanoic acid with high consistency and robustness.
The scalability of the route has been validated beyond laboratory scale. The process was successfully demonstrated at a 25 kg scale, produced in 5 kg batches, confirming its suitability for practical manufacturing environments and downstream integration.
Beyond scale-up, the work also highlights the potential for process intensification. A proof of principle was demonstrated in which the first three reaction steps were telescoped, reducing intermediate handling and opening the door to more streamlined and efficient production workflows. Although this approach afforded the target compound in an isolated yield and quality comparable to those achieved in earlier campaigns, it was more cost-efficient and environmentally friendly. This philosophy reflects InnoSyn’s mindset of continuously optimizing processes to reduce costs and minimize environmental impact.
READ THE PUBLICATION